 02.06.2021
02.06.2021
Why are lotus flowers always clean?
In the late 90’s a poetically titled article “Purity of the sacred lotus, or escape from contamination in biological surfaces”[1] stirred the academic society. The measurements of various water plants showed that there is an undeniable link between the topography of the surface and its ability to repel water.
This started a vivid interest in how the surface topography may shape the properties of the material. Soon many other examples of similar effects were found. For example, the adhesive properties of geckos skin, or the diffractive surfaces of butterfly wings.
When it comes to the lotus flowers the answer lays in the surface having hydrophobic properties[2]. Called as such from the Greek words hydro (ang. water) and phobia (ang. fear) means that water is repelled by the flower’s petals. Thanks to that the flowers “clean themselves”. Every splash of water on its surface takes away any contamination and easily slides off the petals.
That was where the authors of the initial article stopped. Other scientists – mostly those interested in materials sciences went further. Recreating similar topographies on various materials allowed them to create both hydrophobic and hydrophilic surfaces on metal and glass. The whole trick lies in creating a material with the right static and dynamic contact angle. This is the angle between the water droplets and the surface. The fun part is though that the contact angle is only an experimental term – as of now, there is no widely accepted theory why those exact surfaces repel or attract water this way.
The lack of theory didn’t yet stop anyone from finding many practical applications for hydrophobic surfaces. It found use in the creation of self-cleaning windows, anti-stain and anti-microbial medical tools and surfaces, luxurious knives, and microfluidic chips. Recently it was even used to create metal that can float on water. For more information on that see this article: https://pubs.acs.org/doi/10.1021/acsami.9b15540
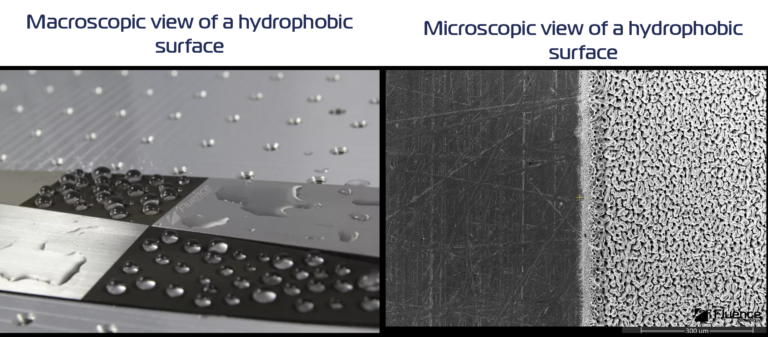
Particularly interesting is to see the surface under the SEM microscope. There you can see the unprocessed steel which is fairly smooth. On the other side, there’s a rough hydrophobic surface with a characteristic pattern of small holes. If you’re interested in even more visual illustration check out our video showing how the water slides off the hydrophobic surfaces.
One of the ways to create such surfaces is through laser surface structuring. With the help of our industrial-grade Jasper femtosecond laser, we created some hydrophobic samples.
Contact us for more information!
[1] Barthlott, W., Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202, 1–8 (1997).
[2] Law, Kock-Yee, “Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right”, J. Phys. Chem. Lett. 2014, 5, 4, 686–688